In a world of regulatory compliance, there has never been a more important time for the healthcare industry to address product information management.
Patient safety, medical device traceability, and government regulations are having a profound effect on product information within the healthcare industry. The demands are growing and it is essential that healthcare companies find a comprehensive and cost-effective approach to manage, collect, validate and distribute information.
1WorldSync Healthcare Solution
Serving more than 3,500 organizations around the globe managing around 2.2 million products in the healthcare sector alone, 1WorldSync is the leading expert for product content solutions in the healthcare industry.
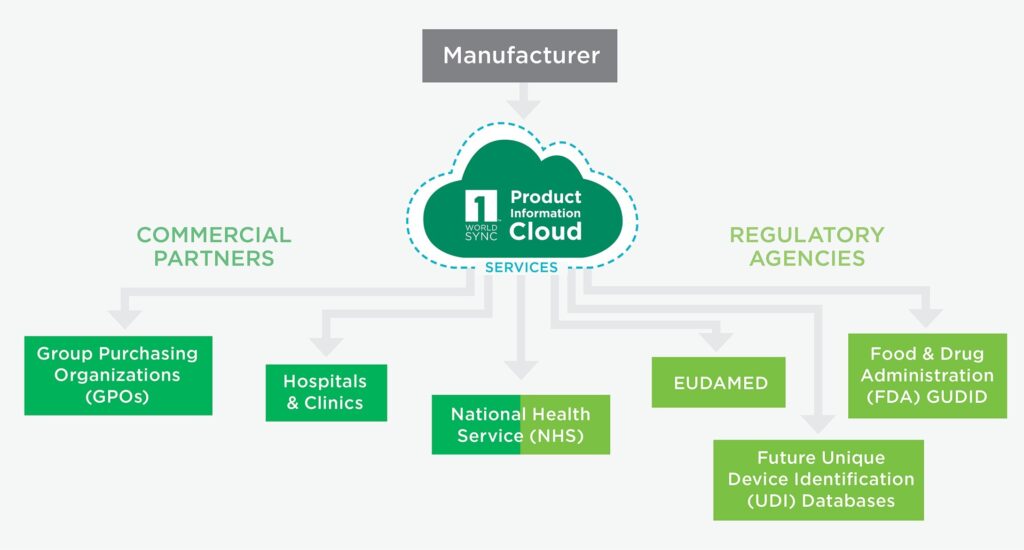
Our healthcare solution is specifically designed to manage and publish Unique Device Identification (UDI) data to healthcare authorities (such as EUDAMED, FDA GUDID) and all necessary commercial data to commercial partners (such as hospitals, clinics, NHS, and group purchasing organizations) worldwide. It allows medical device manufacturers to distribute validated and authentic UDI and commercial data in compliance with different regional requirements.
As future UDI regulations develop and more projects regarding product data exchange mature, 1WorldSync will continue to expand our healthcare solution to fit manufacturers’ regulatory and commercial needs.
The Comprehensive Regulatory and Commercial Solution for Healthcare
The Benefits of the 1WorldSync Healthcare Solution
- One single solution covering all your needs
- Compliance with all regulatory authorities
- Efficiency in managing and publishing your product data
- Anticipation of future UDI requirements
- Security due to ISO Certification 27001
- Validation of your data
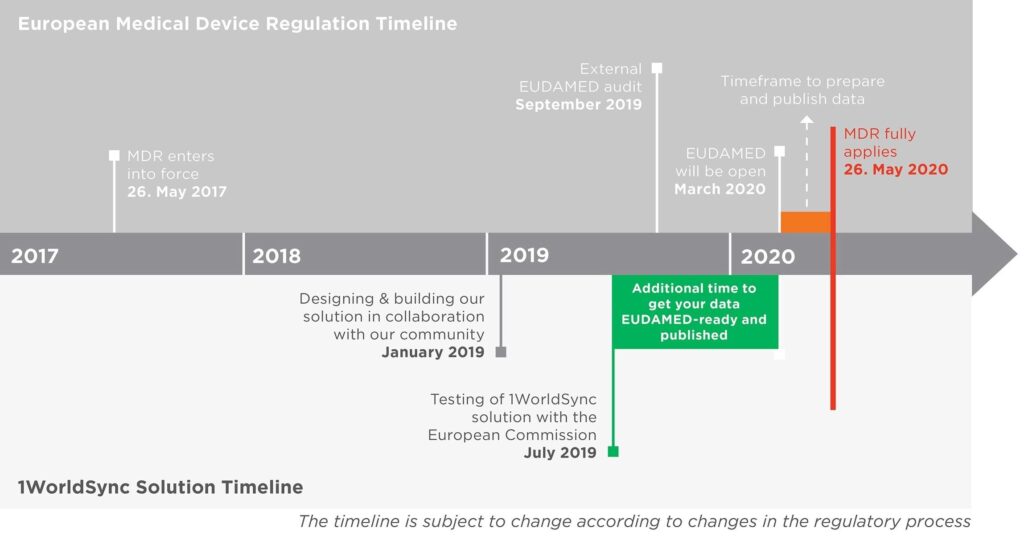
The European Medical Device Regulation & EUDAMED
The European Medical Device Regulation (EU MDR) ensures high standards of quality and safety for medical devices being produced in or supplied into Europe. It will establish a robust, transparent, predictable and sustainable regulatory framework for medical devices to ensure a high level of health and safety whilst supporting innovation by establishing a European UDI database – EUDAMED. EU MDR is relevant to any organization producing or supplying medical device products to the EU.
The MDR & 1WorldSync Solution Timeline
The EUDAMED Connection
1WorldSync’s connection to EUDAMED leverages the existing, regulatory connections, such as FDA GUDID and the NHS connections, and combines it with the existing GDSN expertise between healthcare trading partners. Our healthcare solution was developed and is continuously expanded and adapted to the changing regulatory and commercial needs in close collaboration with our active community of healthcare manufacturers, providers, GPOs and associations (such as MedTech Europe). Through their commitment and dedication, 1WorldSync was able to build and maintain a comprehensive regulatory and commercial solution for all healthcare manufacturers.